A New Paradigm in
Drug Discovery
AlphaGen Bio is a preclinical-stage biotechnology company developing first-in-class therapeutics that harness the power of metabolic reprogramming to treat neurodegenerative diseases.
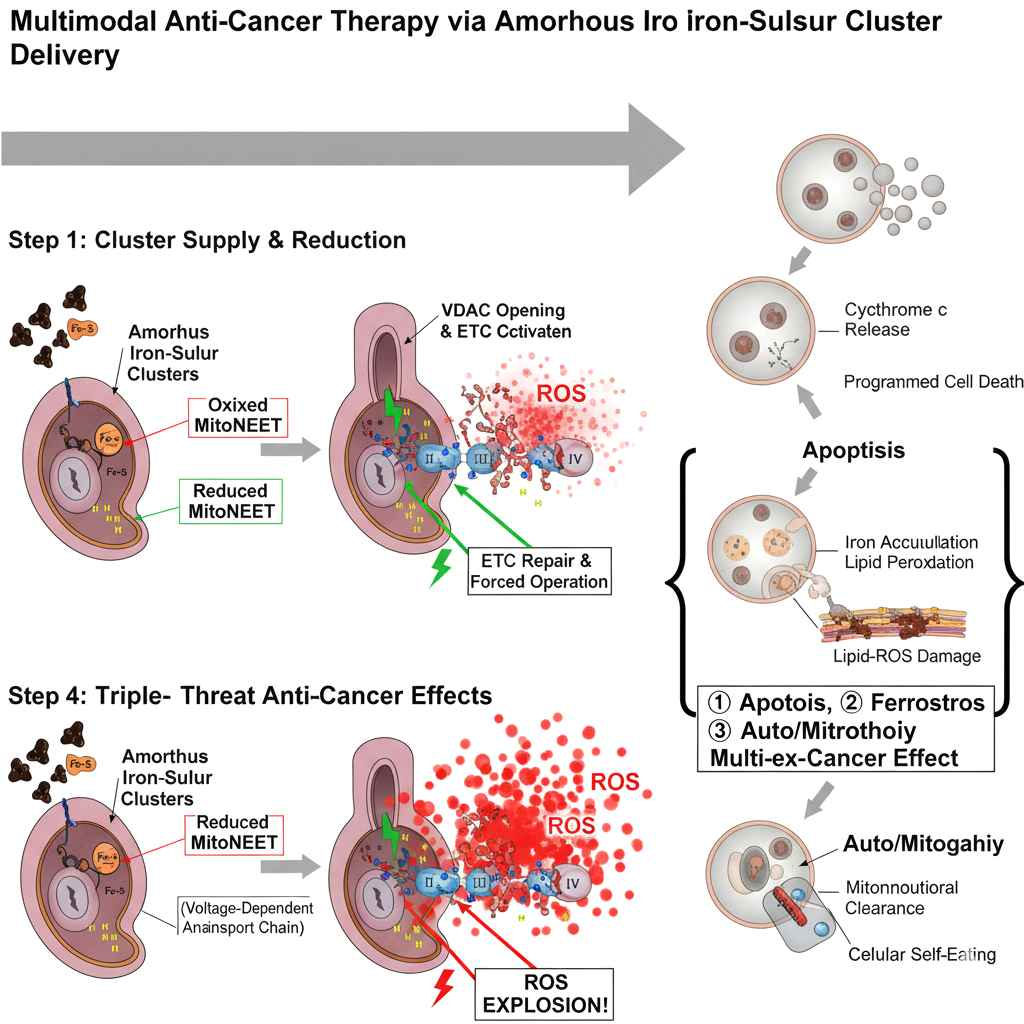
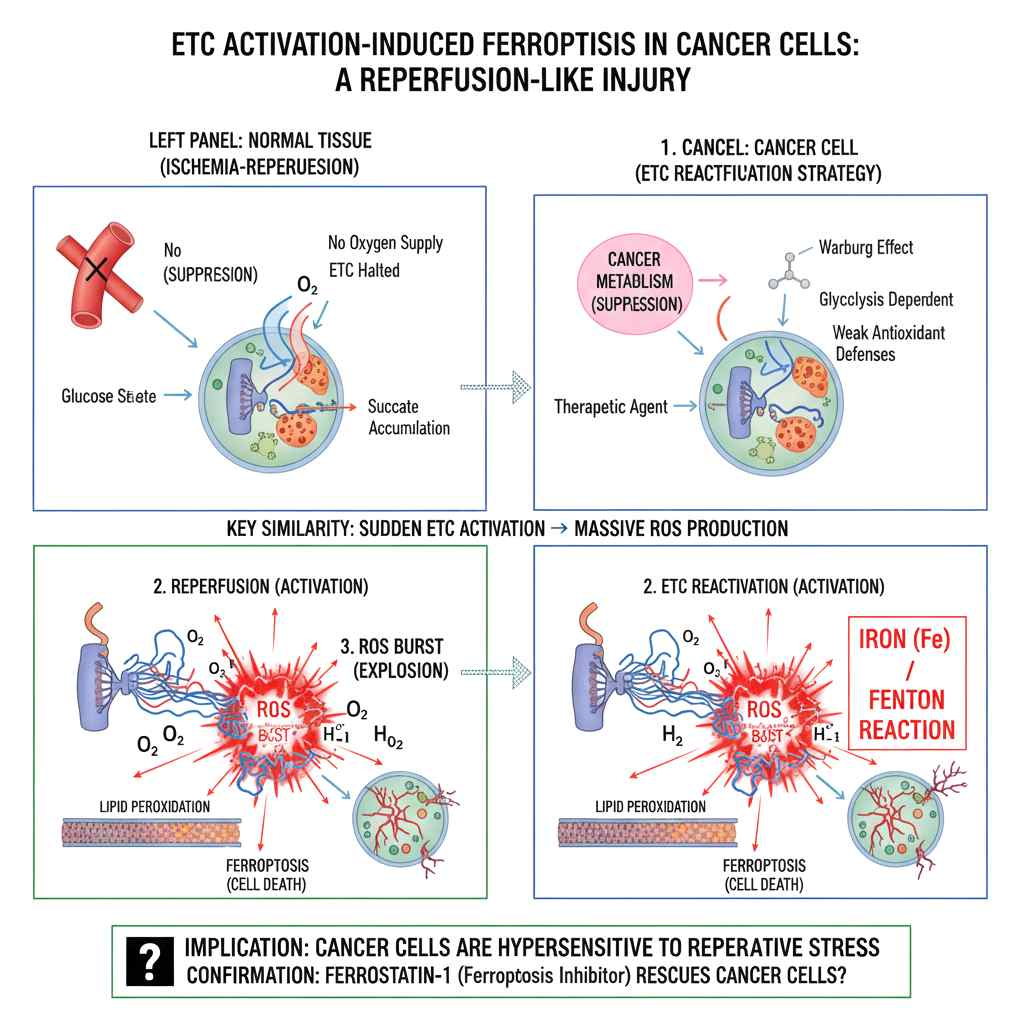
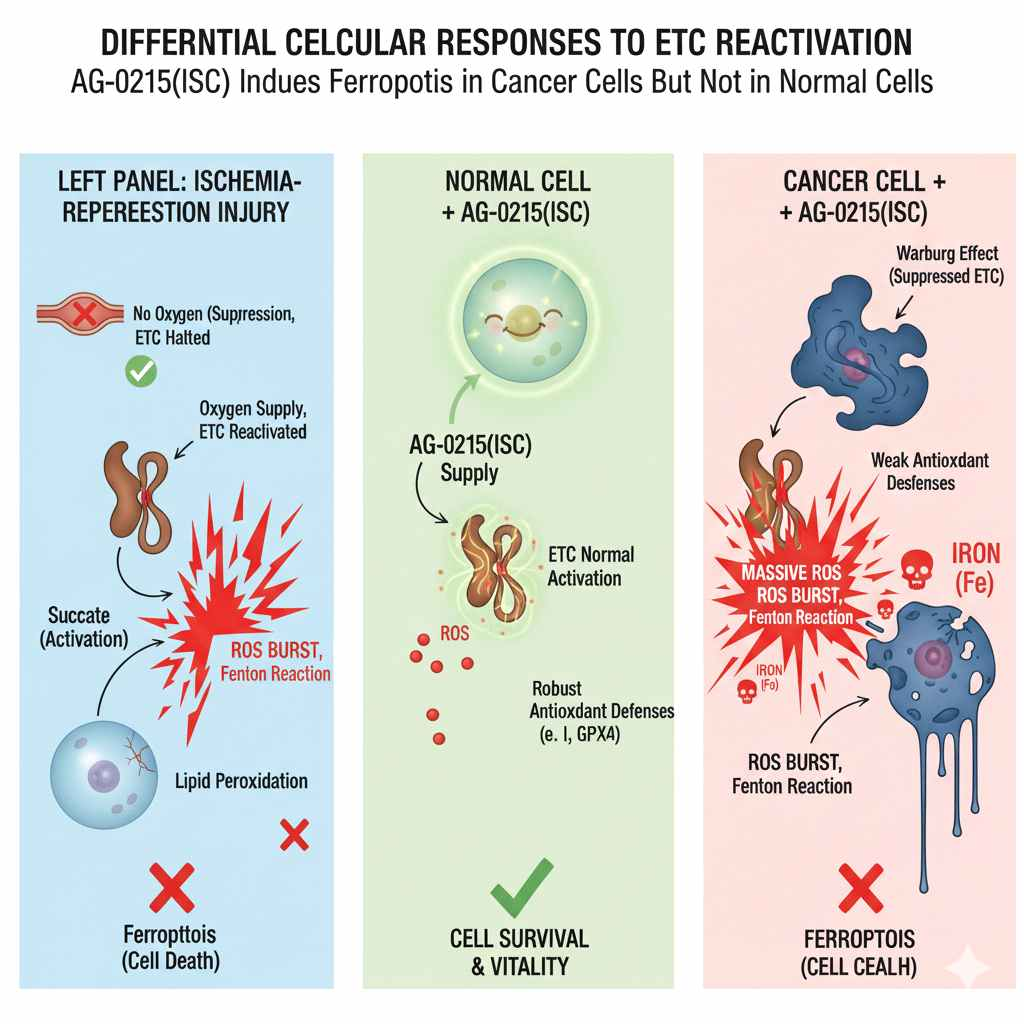
Our proprietary platform is built on the discovery that Metabolism-Based Ferroptosis — a fundamentally different mechanism from traditional chemical ferroptosis — can selectively target and eliminate pathological cells based on their metabolic state, while preserving healthy tissue.
By targeting the MINT protein (CISD3/MiNT), a mitochondrial iron-sulfur cluster protein, our lead compound AG-0215 restores mitochondrial function in healthy cells and triggers metabolic-reperfusion ferroptosis in abnormal cells such as pro-inflammatory M1 microglia.